POST TRANSLATIONAL MODIFICATION
Post Translational Modification
Protein after or during translation undergo several modifications to become functional, which are discussed as under:
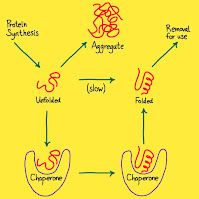
- During and after its synthesis a polypeptide chain begin to coil and fold spontaneously forming a functional protein of specific conformation. A gene determines primary structure, and primary structure in turn determines conformation. In many cases, chaperone proteins help the polypeptide to fold correctly.
- Additional steps such as post-translational modifications may be required before the protein can begin doing its particular job in the cell. Enzymes may remove one or more amino acids from the leading (amino) end of the polypeptide chain .In some cases, a single polypeptide chain may be enzymatically cleaved into two or more pieces. For examples, the protein insulin is first synthesized as a single polypeptide chain, but becomes active only after an enzyme exercise a central part of the chain leaving a protein made up of two polypeptides chains connected by disulfide bridges. In micrographs of eukaryotes cells active in protein synthesis, two populations of ribosomes (and polyribosomes), free and bound are evident. Free ribosomes are suspended in the cytosol, and mostly synthesize proteins that dissolve in the cytosol and function there. In contrast ,bound ribosomes are attached to the cytosolic side of the endoplasmic reticulum (ER). They make proteins of the endomembrane system (the nuclear envelope, ER, Golgi apparatus, lysosomes, vacuoles and plasma membranes) and protein that are secreted from the cell. Insulin is an example of a secretary protein. The ribosomes themselves are identical and can switch their status from free to bound .
- The synthesis of all proteins begins in the cytosol, when a free ribosomes starts to translate an mRNA molecules. There the process continues to completion unless the growing polypeptide itself cues the ribosomes to attach to the ER. The signal peptide, a sequences of about 20 amino acids at or near the leading (amino) end of the polypeptide, is recognized as it emerges from the ribosomes by a protein. RNA complex called a signal-recognition particle(SRP). This particle functions as an adaptor that brings the ribosomes to a receptor protein built into the ER membrane. This receptor is part of a multiprotein complex. Polypeptide synthesis continues there, and the growing polypeptide snakes across the membrane into the cisternal space.
- Other kinds of signal peptides are used to target polypeptides to mitochondria, chloroplasts the interior of the nucleus and other organelles that are not part of the endomembrane system. The mechanisms of translocation also vary, but in all cases studied to date, the "postal" codes that are address proteins to cellular locations are signal peptides of some sort.



Comments
Post a Comment